ASTM F2267-24
(Test Method)Standard Test Method for Measuring Load-Induced Subsidence of Intervertebral Body Fusion Device Under Static Axial Compression
Standard Test Method for Measuring Load-Induced Subsidence of Intervertebral Body Fusion Device Under Static Axial Compression
SIGNIFICANCE AND USE
5.1 Intervertebral body fusion devices are generally simple geometric-shaped devices, which are often porous or hollow in nature. Their function is to support the anterior column of the spine to facilitate arthrodesis of the motion segment.
5.2 This test method is designed to quantify the subsidence characteristics of different designs of intervertebral body fusion devices since this is a potential clinical failure mode. These tests are conducted in vitro in order to simplify the comparison of simulated vertebral body subsidence induced by the intervertebral body fusion devices.
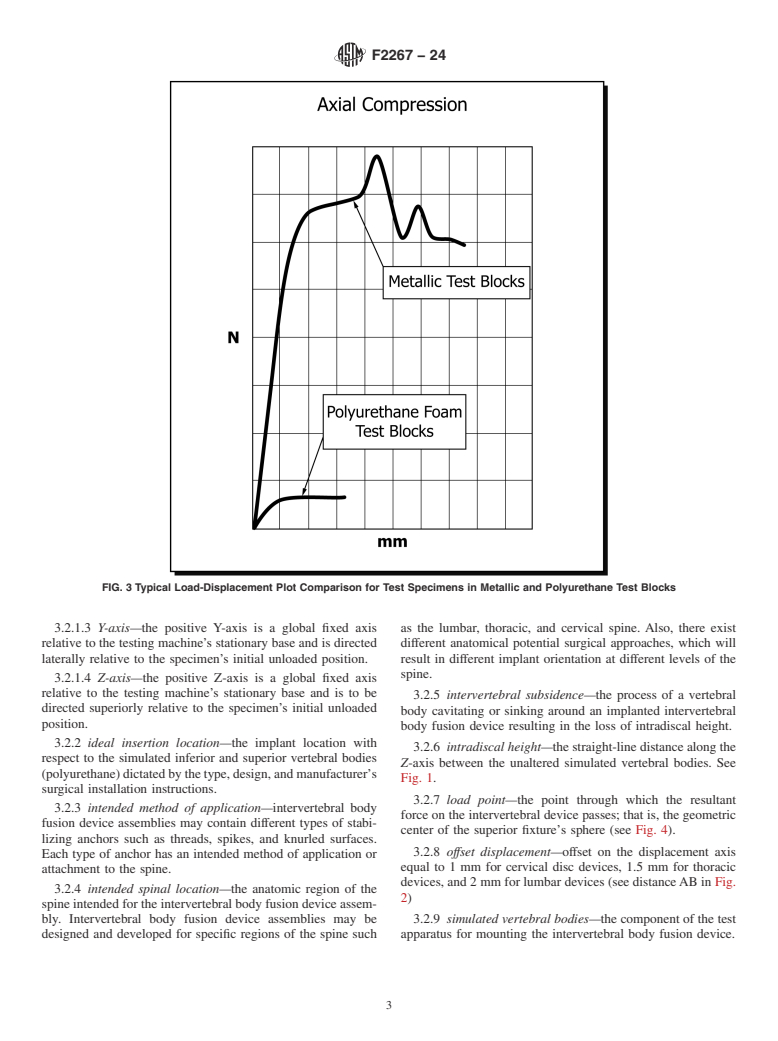
5.3 The static axial compressive loads that will be applied to the intervertebral body fusion devices and test blocks will differ from the complex loading seen in vivo, and therefore, the results from this test method may not be used to directly predict in vivo performance. The results, however, can be used to compare the varying degrees of subsidence between different intervertebral body fusion device designs for a given density of simulated bone.
5.4 The location within the simulated vertebral bodies and position of the intervertebral body fusion device with respect to the loading axis will be dependent upon the design and manufacturer's recommendation for implant placement.
SCOPE
1.1 This test method specifies the materials and methods for the axial compressive subsidence testing of non-biologic intervertebral body fusion devices, spinal implants designed to promote arthrodesis at a given spinal motion segment.
1.2 This test method is intended to provide a basis for the mechanical comparison among past, present, and future non-biologic intervertebral body fusion devices. This test method is intended to enable the user to mechanically compare intervertebral body fusion devices and does not purport to provide performance standards for intervertebral body fusion devices.
1.3 This test method describes a static test method by specifying a load type and a specific method of applying this load. This test method is designed to allow for the comparative evaluation of intervertebral body fusion devices.
1.4 Guidelines are established for measuring test block deformation and determining the subsidence of intervertebral body fusion devices.
1.5 Since some intervertebral body fusion devices require the use of additional implants for stabilization, the testing of these types of implants may not be in accordance with the manufacturer's recommended usage.
1.6 Units—The values stated in SI units are to be regarded as the standard with the exception of angular measurements, which may be reported in terms of either degrees or radians.
1.7 The use of this standard may involve the operation of potentially hazardous equipment. This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.8 This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
General Information
Relations
Buy Standard
Standards Content (Sample)
This international standard was developed in accordance with internationally recognized principles on standardization established in the Decision on Principles for the
Development of International Standards, Guides and Recommendations issued by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
Designation: F2267 − 24
Standard Test Method for
Measuring Load-Induced Subsidence of Intervertebral Body
1
Fusion Device Under Static Axial Compression
This standard is issued under the fixed designation F2267; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope ization established in the Decision on Principles for the
Development of International Standards, Guides and Recom-
1.1 This test method specifies the materials and methods for
mendations issued by the World Trade Organization Technical
the axial compressive subsidence testing of non-biologic in-
Barriers to Trade (TBT) Committee.
tervertebral body fusion devices, spinal implants designed to
promote arthrodesis at a given spinal motion segment.
2. Referenced Documents
2
1.2 This test method is intended to provide a basis for the
2.1 ASTM Standards:
mechanical comparison among past, present, and future non-
E4 Practices for Force Calibration and Verification of Test-
biologic intervertebral body fusion devices. This test method is
ing Machines
intended to enable the user to mechanically compare interver-
E177 Practice for Use of the Terms Precision and Bias in
tebral body fusion devices and does not purport to provide
ASTM Test Methods
performance standards for intervertebral body fusion devices.
E691 Practice for Conducting an Interlaboratory Study to
Determine the Precision of a Test Method
1.3 This test method describes a static test method by
F1839 Specification for Rigid Polyurethane Foam for Use as
specifying a load type and a specific method of applying this
a Standard Material for Testing Orthopaedic Devices and
load. This test method is designed to allow for the comparative
Instruments
evaluation of intervertebral body fusion devices.
F2077 Test Methods for Intervertebral Body Fusion Devices
1.4 Guidelines are established for measuring test block
deformation and determining the subsidence of intervertebral 3. Terminology
body fusion devices.
3.1 All subsidence testing terminology is consistent with the
1.5 Since some intervertebral body fusion devices require referenced standards above, unless otherwise stated.
the use of additional implants for stabilization, the testing of
3.2 Definitions:
these types of implants may not be in accordance with the
3.2.1 coordinate system/axes—three orthogonal axes are
manufacturer’s recommended usage.
defined following a right-handed Cartesian coordinate system
1.6 Units—The values stated in SI units are to be regarded (Fig. 4). The XY plane bisects the sagittal plane between the
as the standard with the exception of angular measurements, superior and inferior surfaces that are intended to simulate the
which may be reported in terms of either degrees or radians. adjacent vertebral end plates. The positive Z-axis is to be
directed superiorly. Force components parallel to the XY plane
1.7 The use of this standard may involve the operation of
are shear components of loading. The compressive axial force
potentially hazardous equipment. This standard does not pur-
is defined to be the component in the negative Z direction.
port to address all of the safety concerns, if any, associated
Torsional load is defined to be the component of moment about
with its use. It is the responsibility of the user of this standard
the Z-axis.
to establish appropriate safety, health, and environmental
practices and determine the applicability of regulatory limita- 3.2.1.1 origin—the center of the coordinate system is lo-
tions prior to use. cated at the center of rotation of the testing fixture.
1.8 This international standard was developed in accor-
3.2.1.2 X-axis—the positive X-axis is a global fixed axis
dance with internationally recognized principles on standard-
relative to the testing machine’s stationary base and is to be
directed anteriorly relative to the specimen’s initial unloaded
position.
1
This test method is under the jurisdiction of ASTM Committee F04 on Medical
and Surgical Materials and Devices and is the direct responsibility of Subcommittee
2
F04.25 on Spinal Devices. For referenced ASTM standards, visit the ASTM website, www.astm.org, or
Current edition approved April 15, 2024. Published April 2024. Originally contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM
approved in 2003. Last previous edition approved in 2022 as F2267 – 22. DOI: Standards volume information, refer to the standard’s Document Summary page on
10.1520/F2267-
...
This document is not an ASTM standard and is intended only to provide the user of an ASTM standard an indication of what changes have been made to the previous version. Because
it may not be technically possible to adequately depict all changes accurately, ASTM recommends that users consult prior editions as appropriate. In all cases only the current version
of the standard as published by ASTM is to be considered the official document.
Designation: F2267 − 22 F2267 − 24
Standard Test Method for
Measuring Load-Induced Subsidence of Intervertebral Body
1
Fusion Device Under Static Axial Compression
This standard is issued under the fixed designation F2267; the number immediately following the designation indicates the year of
original adoption or, in the case of revision, the year of last revision. A number in parentheses indicates the year of last reapproval. A
superscript epsilon (´) indicates an editorial change since the last revision or reapproval.
1. Scope
1.1 This test method specifies the materials and methods for the axial compressive subsidence testing of non-biologic
intervertebral body fusion devices, spinal implants designed to promote arthrodesis at a given spinal motion segment.
1.2 This test method is intended to provide a basis for the mechanical comparison among past, present, and future non-biologic
intervertebral body fusion devices. This test method is intended to enable the user to mechanically compare intervertebral body
fusion devices and does not purport to provide performance standards for intervertebral body fusion devices.
1.3 This test method describes a static test method by specifying a load type and a specific method of applying this load. This test
method is designed to allow for the comparative evaluation of intervertebral body fusion devices.
1.4 Guidelines are established for measuring test block deformation and determining the subsidence of intervertebral body fusion
devices.
1.5 Since some intervertebral body fusion devices require the use of additional implants for stabilization, the testing of these types
of implants may not be in accordance with the manufacturer’s recommended usage.
1.6 Units—The values stated in SI units are to be regarded as the standard with the exception of angular measurements, which may
be reported in terms of either degrees or radians.
1.7 The use of this standard may involve the operation of potentially hazardous equipment. This standard does not purport to
address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish
appropriate safety, health, and environmental practices and determine the applicability of regulatory limitations prior to use.
1.8 This international standard was developed in accordance with internationally recognized principles on standardization
established in the Decision on Principles for the Development of International Standards, Guides and Recommendations issued
by the World Trade Organization Technical Barriers to Trade (TBT) Committee.
2. Referenced Documents
2
2.1 ASTM Standards:
1
This test method is under the jurisdiction of ASTM Committee F04 on Medical and Surgical Materials and Devices and is the direct responsibility of Subcommittee
F04.25 on Spinal Devices.
Current edition approved Sept. 1, 2022April 15, 2024. Published September 2022April 2024. Originally approved in 2003. Last previous edition approved in 20182022
as F2267 – 04 (2018).F2267 – 22. DOI: 10.1520/F2267-22.10.1520/F2267-24.
2
For referenced ASTM standards, visit the ASTM website, www.astm.org, or contact ASTM Customer Service at service@astm.org. For Annual Book of ASTM Standards
volume information, refer to the standard’s Document Summary page on the ASTM website.
Copyright © ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA 19428-2959. United States
1
---------------------- Page: 1 ----------------------
F2267 − 24
E4 Practices for Force Calibration and Verification of Testing Machines
E177 Practice for Use of the Terms Precision and Bias in ASTM Test Methods
E691 Practice for Conducting an Interlaboratory Study to Determine the Precision of a Test Method
F1582 Terminology Relating to Spinal Implants
F1839 Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and
Instruments
F2077 Test Methods for Intervertebral Body Fusion Devices
3. Terminology
3.1 All subsidence testing terminology is consistent with the referenced standards above, unless otherwise stated.
3.2 Definitions:
3.2.1 coordinate system/axes—three orthogonal axes are defined by Terminologyfollowing a F1582 as seen inright-handed
Cartesian coordinate system (Fig. 4. The center of the coordinate system is located at the geometric center of the intervertebral
body fusion device assembly. ). The XY plane bisects the sagittal plane between the superior and inferior surfaces that are intended
to simulate the adjacent vertebral end plates. T
...








Questions, Comments and Discussion
Ask us and Technical Secretary will try to provide an answer. You can facilitate discussion about the standard in here.